Physical and Chemical Properties of Minerals
The Physical and Chemical Properties of Minerals are the characteristics that help in identifying and understanding minerals. Physical properties include observable traits like color, luster, hardness, cleavage, and density. Chemical properties refer to the mineral's composition and reactivity, such as its chemical formula, solubility, and response to acids. These properties can be used to identify, classify, and study of minerals.
 |
| Physical and Chemical Properties of Minerals Chart. |
Physical Properties of Minerals
Physical properties of minerals are the characteristics that can be observed or measured without changing the mineral’s chemical composition. These properties are based on the physical arrangement of atoms, the crystal structure, and the forces that hold the structure together. They help identify and differentiate minerals and are easily tested using common methods. Here are some key physical properties of minerals:
Color
Mineral Color refers to the visual appearance of a mineral when it reflects or absorbs certain wavelengths of visible light. It’s often the first property noticed, but it can sometimes be unreliable for identification.
While color is the most obvious feature, many minerals display a wide variety of colors due to impurities or weathering. This makes color alone an unreliable identification tool in many cases. However, for some minerals, the color is diagnostic and consistent.
- Chemical Composition: The specific elements in a mineral can give it distinct colors. For example, the green color of malachite is due to its copper content.
- Impurities: Trace elements or impurities can change the color. For instance, pure quartz is colorless, but with impurities, it can appear pink (rose quartz), purple (amethyst), or smoky (smoky quartz).
- Crystal Defects: Structural defects in the crystal lattice can affect how light interacts with the mineral, changing its color.
- Oxidation: Oxidation states of certain metals in a mineral can dramatically affect the color. Hematite appears red or reddish-brown due to the oxidation of iron.
 |
Different colors of minerals found in nature. |
Mineral color Examples:
- Malachite: Always green due to copper.
- Azurite: Deep blue due to copper.
- Sulfur: Yellow due to its sulfur content.
- Quartz: Colorless, but impurities can make it pink (rose quartz), purple (amethyst), or smoky (smoky quartz).
Limitations: Many minerals come in multiple colors, and environmental conditions or chemical impurities can alter the color, making it an inconsistent property for identification on its own.
Transparency
Transparency refers to how much light passes through a mineral. It is a measure of the mineral’s optical properties and how much light can be transmitted through it.
Transparency is important for both identifying minerals and determining their uses, particularly in the fields of optics and jewelry.
How to observe it: Transparency is observed by holding the mineral up to a light source and examining how much light passes through and how clear the view is through the mineral.
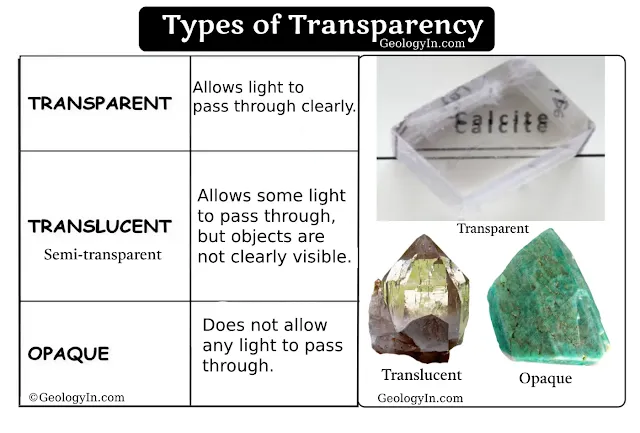 |
| Mineral Types of Transparency: Transparent, Translucent and Opaque |
Types of Transparency:
Transparent: Light passes through the mineral without significant distortion, and objects can be seen clearly through it. Example: Quartz and calcite can be transparent when in pure form.
Translucent: Light passes through the mineral, but objects cannot be clearly distinguished. The mineral allows light through but scatters it, making objects appear blurry. Example: Milky quartz, opal, and gypsum are examples of translucent minerals.
Opaque: No light passes through the mineral; it is completely non-transparent. Example: Galena, hematite, and pyrite are opaque minerals.
Streak
Mineral Streak refers to the color of a mineral in its powdered form, which is obtained by rubbing the mineral on an unglazed porcelain streak plate.
Streak is often more reliable than the color of the mineral itself because the powdered form is less affected by impurities and structural defects. This property is particularly useful for identifying minerals that exhibit metallic luster, as their streak color can be very different from their surface color.
How to observe it: The streak test involves rubbing the mineral across a streak plate (usually unglazed porcelain) to see the color of the powder left behind. It’s important to note that only minerals softer than the streak plate (about 6.5 on the Mohs hardness scale) will leave a streak.
 |
Mineral streak tests showing the color of the mineral powder |
Streak Colors:
- Metallic Minerals: Generally have a dark streak (black, brown, red).
- Non-metallic Minerals: Usually have a light or colorless streak.
Examples of Mineral Streaks:
- Hematite: Often has a metallic gray color but leaves a reddish-brown streak.
- Pyrite: Known as "fool's gold" for its gold-like color, it leaves a blackish-green streak.
- Galena: Silvery-gray in color, but its streak is black.
- Quartz: Being harder than the streak plate, it does not leave a streak.
Importance: Since streak color is more consistent than the surface color of a mineral, it is a crucial identification tool, particularly for minerals with metallic luster or for those whose surface color varies due to weathering or impurities.
Luster
Mineral Luster refers to the way light interacts with the surface of a mineral. It describes the appearance or quality of light reflected from the mineral’s surface, and it can be a good indicator of a mineral's identity.
How to observe it: The luster of a mineral is assessed by observing how it reflects light. It can be observed under natural light or a bright artificial light source.
 |
| Types of mineral Luster: metallic and non-metallic. like vitreous, adamantine, resinous, pearly, greasy, silky, and earthy. |
Metallic Luster: Reflects light like metal. These minerals are usually opaque and shiny.
Non-metallic Luster: Minerals that do not appear metallic. This category includes several subtypes:
- Vitreous (Glassy): Reflects light like glass. Example: Quartz.
- Pearly: Has a pearl-like sheen. Example: Talc, muscovite.
- Resinous: Looks like resin or amber. Example: Sphalerite.
- Silky: Has a sheen similar to silk. Example: Fibrous minerals like asbestos and gypsum.
- Greasy: Appears as though coated with oil. Example: Graphite, some forms of quartz.
- Dull (Earthy): Lacks shine, looks like soil or clay. Example: Kaolinite.
- Adamantine: Exhibits a brilliant, diamond-like shine. Example: Diamond.
- Waxy: Appears to have a waxy surface. Example: Opal.
Hardness
Hardness is the measure of a mineral’s resistance to being scratched. It reflects the strength of the atomic bonds within the mineral structure and is one of the most commonly tested physical properties of minerals.
Hardness is a critical diagnostic tool for mineral identification. It is relatively easy to test in the field and in laboratories and provides valuable information about the mineral's durability and potential uses.
How to Measure Hardness: Hardness is most commonly measured using Mohs Hardness Scale, developed by Friedrich Mohs in 1812. This scale ranks minerals on a scale from 1 (softest) to 10 (hardest) by comparing a mineral's ability to scratch another material or be scratched by it. The hardness of unknown minerals can be estimated by scratching them with known substances.
 |
Mohs Hardness Scale is a relative measure of the scratch resistance of minerals. It ranges from 1 (softest) to 10 (hardest). |
Mohs Hardness Scale:
Talc – Softest mineral; easily scratched with a fingernail.
Gypsum – Can be scratched by a fingernail.
Calcite – Scratched by a copper coin.
Fluorite – Scratched by steel or a knife.
Apatite – Harder than steel but can be scratched by a file.
Orthoclase (Feldspar) – Can scratch glass.
Quartz – Hard enough to scratch glass and steel.
Topaz – Can scratch quartz.
Corundum – Can scratch topaz and nearly all other minerals except diamond.
Diamond – Hardest known mineral; can scratch any other material.
Cleavage
Mineral Cleavage refers to the tendency of a mineral to break along flat, even surfaces, which are determined by the mineral’s crystal structure. These cleavage planes are areas of weakness in the atomic bonding, where the mineral breaks more easily.
Cleavage is a highly diagnostic property of minerals because it reveals how a mineral’s internal atomic structure is organized. The way a mineral cleaves can help identify it, as different minerals have different numbers and orientations of cleavage planes.
How to observe it: Cleavage is observed by examining how a mineral breaks. To test for cleavage, a small portion of the mineral can be broken, and the resulting surfaces are examined to see if they form smooth, flat planes.
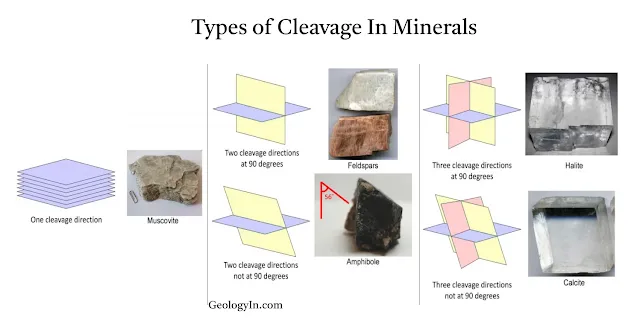 |
| Types of Cleavage in minerals. |
Minerals can have one or more directions of cleavage, depending on their crystal structure. Cleavage directions are described by the angles between the planes of cleavage.
Types of Cleavage Based on Cleavage Directions
The number of cleavage planes and the angles between them are important for identifying minerals. Common types include:
Basal (1 Direction): The mineral splits into thin sheets along one plane.Example: Mica (e.g., biotite, muscovite) has perfect basal cleavage, splitting into thin, flexible sheets.
Prismatic (2 Directions): Breaks into prismatic shapes along two cleavage directions. Example: Feldspar (e.g., orthoclase) has prismatic cleavage, with two cleavage directions at nearly right angles.
Cubic (3 Directions at 90°): The mineral breaks into cubes due to three directions of cleavage intersecting at right angles. Example: Halite (salt) and galena exhibit cubic cleavage.
Rhombohedral (3 Directions not at 90°): Cleavage planes intersect but at angles other than 90°, forming rhombohedral shapes. Example: Calcite has rhombohedral cleavage.
Octahedral (4 Directions): Breaks into shapes with eight faces, due to four directions of cleavage. Example: Fluorite exhibits octahedral cleavage.
Describing Cleavage Quality
- Perfect Cleavage: The mineral splits easily along one or more planes with smooth, flat surfaces (e.g., mica).
- Good Cleavage: Breaks along cleavage planes with relatively smooth surfaces but may not be perfect (e.g., feldspar).
- Poor or Indistinct Cleavage: Cleavage planes are not easily seen, or the mineral breaks irregularly (e.g., apatite).
Examples:
- Mica: Exhibits perfect cleavage in one direction, allowing it to split into thin sheets.
- Calcite: Cleaves along three planes not at right angles, producing rhombohedral fragments.
- Halite: Breaks into cubes due to its cubic cleavage.
Fracture
Mineral Fracture refers to the way a mineral breaks when it does not follow cleavage planes. Fracture occurs when the bonding forces between the atoms are equally strong in all directions, and it results in an irregular or non-planar breakage surface.
Unlike cleavage, which occurs along specific planes, fracture occurs in minerals that either lack cleavage or break irregularly. The type of fracture is often useful in identifying minerals that do not exhibit cleavage or that break in more complex ways.
How to observe it: Fracture can be tested by breaking or chipping the mineral in a way that does not follow any cleavage planes. The resulting surface is then observed to determine the type of fracture.
 |
| Types of Fracture in minerals: Include conchoidal (curved), hackly (jagged), and splintery (long, thin pieces). |
Types of Fracture:
Conchoidal Fracture: Smooth, curved surfaces that resemble the interior of a shell. This type of fracture is common in minerals with no cleavage, and it often occurs in very hard, brittle minerals. Examples: Quartz, obsidian (volcanic glass).
Splintery Fracture: or Fibrous Breaks into fibers or splinters, resembling wood or fibrous material. Examples: Asbestos, serpentine.
Hackly Fracture: Jagged, sharp, and torn surfaces, often resembling broken metal, often with sharp edges. This type of fracture is common in native metals. Examples: Native copper, native silver.
Uneven Fracture: Rough, irregular surfaces. This is the most common type of fracture and occurs in many minerals. Examples: Hematite, pyrite.
Earthy Fracture: Breaks with a dull, powdery surface, often seen in soft, fine-grained minerals. Examples: Limonite, kaolinite.
Form (Habit)
Crystal form, or habit, refers to the external shape that a mineral's crystals assume when they have enough space to grow uninhibited. This form is a direct reflection of the mineral’s internal atomic structure and the symmetry of its crystal lattice.
Crystal habit helps to identify minerals, especially when they are well-formed. The habit is influenced by the conditions of growth, such as temperature, pressure, and the presence of space for unrestricted crystal formation.
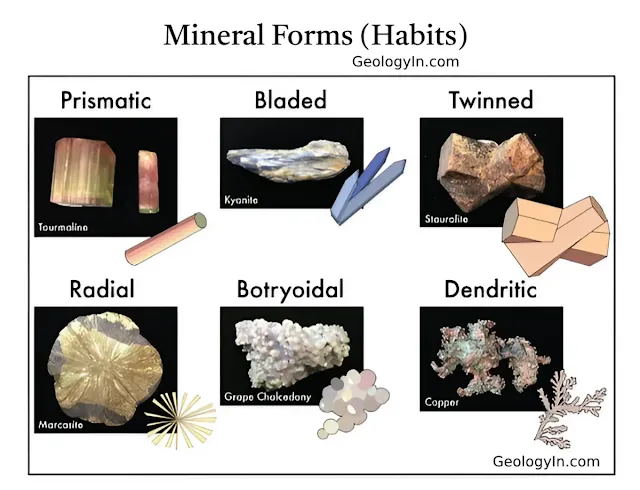 |
Mineral forms and habits: granular, prismatic, acicular, dendritic, botryoidal, granular, and massive. |
Equant (Cubic): Crystals that are roughly equal in all dimensions, giving them a blocky or cubic appearance. Example: Garnet forms equant, dodecahedral crystals.
Tabular: Crystals that are flat and plate-like, resembling a table. Example: Barite and gypsum often exhibit a tabular habit.
Prismatic: Elongated crystals that are much longer in one direction than in others, often forming prism-like shapes. Example: Quartz typically forms prismatic crystals with hexagonal cross-sections.
Acicular: Needle-like crystals that are thin and long, forming slender points. Example: Natrolite and other zeolite minerals often exhibit acicular habits.
Bladed: Thin, flat crystals that resemble the shape of a knife blade. Example: Kyanite forms bladed crystals.
Fibrous: Crystals that grow in long, thread-like strands. Example: Chrysotile (a form of asbestos) exhibits fibrous crystal form.
Botryoidal: Crystals that form rounded, grape-like clusters. Example: Hematite and malachite can form botryoidal masses.
Dendritic: Tree-like or branching crystal formations. Example: Native silver and manganese oxides (e.g., pyrolusite) exhibit dendritic patterns.
Massive: Lacks distinct crystal faces, often forming large, shapeless aggregates.Example: Limonite and chalcedony are examples of minerals with massive habits.
Crystal habit can be observed by examining a mineral specimen closely, particularly if the crystals are well-formed. Ideally, minerals are viewed in samples where they have grown freely without being confined by surrounding materials.
Specific Gravity (Density)
Specific gravity (SG) is the ratio of the weight of a mineral to the weight of an equal volume of water at 4°C. It is a measure of the density of a mineral relative to water, with no units attached.
Specific gravity is an important property in mineral identification because it reflects the mineral’s composition and atomic structure. Minerals with a higher specific gravity are typically composed of heavier elements or have a denser atomic structure.
 |
| Testing mineral specific gravity |
Example: Gold has a high specific gravity (about 19.3), making it feel much heavier than other minerals of the same size. Quartz: SG = 2.65; a common, relatively low-density mineral.
Tenacity
Tenacity describes how a mineral responds to stress, such as bending, breaking, crushing, or pulling. It indicates the mineral’s toughness or resistance to deformation.
Tenacity is an important physical property because it helps determine how a mineral can be used, especially in industrial and manufacturing processes where stress resistance is important.
Types of Tenacity:
Brittle: Minerals that break or shatter easily when struck. They have little resistance to breaking. Example: Quartz, calcite, and halite are brittle and shatter when hit.
Malleable: Minerals that can be hammered into thin sheets without breaking. Example: Gold and copper are malleable, meaning they can be shaped without fracturing.
Ductile: Minerals that can be stretched into a wire without breaking. Example: Gold, copper, and silver exhibit ductility.
Sectile: Minerals that can be cut smoothly with a knife. Example: Gypsum and talc can be cut with a knife due to their softness.
Elastic: Minerals that bend and return to their original shape after the stress is removed. Example: Mica is elastic and can bend without breaking, returning to its original form when released.
Flexible: Minerals that can bend but do not return to their original shape once bent. Example: Chlorite can bend and remain in the bent shape.
Magnetism
Magnetism in minerals refers to their ability to interact with magnetic fields, which includes being attracted to magnets, repelling from them, or the mineral itself generating a magnetic field. It is determined by the arrangement of unpaired electrons in a mineral’s atomic structure. The presence of elements like iron (Fe), nickel (Ni), and cobalt (Co) usually leads to magnetic behavior.
To test observe it a small hand magnet is placed near the mineral to observe attraction or repulsion.
 |
| Certain minerals like Magnetite demonstrating its magnetic properties by attracting metal objects. Photo: Phil Degginger / Science Photo Library |
Types of Magnetism:
Ferromagnetic: Minerals that are strongly attracted to a magnetic field and can retain magnetism even after the field is removed. Example: Magnetite is the most well-known magnetic mineral.
Paramagnetic: Weakly magnetic Minerals that are weakly attracted to a magnetic field but do not retain any magnetism when the external field is removed. Example: Hematite shows weak magnetic properties.
Diamagnetic: Minerals that are weakly repelled by a magnetic field and have no unpaired electrons. All electrons are paired, resulting in no net magnetic moment. Example: Calcite is diamagnetic.
Double Refraction
Double refraction occurs when a ray of light passes through a mineral and splits into two rays, creating a double image when viewed through the mineral. This optical property is due to the difference in how light is refracted in different directions within the crystal.
 |
| Double refraction Image splitting by a calcite crystal. |
How to observe it: Double refraction can be tested by placing a mineral over printed text or a line and observing if the text appears doubled.
Examples:
- Calcite: The most famous example of double refraction. When a clear crystal of calcite is placed over printed text, two images of the text are seen.
- Iceland spar (a form of calcite) exhibits double refraction, where objects viewed through the crystal appear doubled.
Taste
Certain soluble minerals have distinctive tastes, which can help in their identification. The taste is generally tested by carefully placing a small amount of the mineral on the tongue.
 |
| Certain minerals have distinctive tastes like halite. Screenshots of the movie 'Pirates of the Caribbean'. |
Important Note: Taste testing should be done with caution since some minerals may be toxic.
Examples:
- Halite (Rock Salt): Tastes like common table salt (NaCl).
- Sylvite: Tastes bitter compared to halite due to its potassium content (KCl).
Odor / Smell
Certain minerals emit characteristic odors, either when scratched, broken, or exposed to heat or moisture. These odors can help in identifying specific minerals.
How to Test it: Odor can be detected by scratching or moistening the mineral, or by heating it slightly to release volatile compounds.
Examples:
- Sulfur: Minerals containing sulfur, like pyrite, can give off a sulfurous or "rotten egg" odor when rubbed or heated.
- Clay minerals: When moist, some clay minerals, such as kaolinite, may emit a "earthy" or "clay-like" odor.
- Arsenopyrite: When struck or heated, it can release a garlic-like odor due to the release of arsenic fumes.
Tactile or Feel
Certain minerals can have distinct tactile qualities "feels" when touched, helping to identify them.
Examples:
- Smooth: Minerals like selenite (gypsum) or serpentine feel smooth to the touch.
- Greasy: Minerals such as graphite or molybdenite have a greasy feel due to their layer structure.
- Soapy: Talc Feels uniquely soapy due to its extreme softness (Mohs hardness of 1).
- Waxy: Chalcedony or some jaspers can have a waxy feel related to their luster.
- Slippery: Chlorite and graphite feel slippery due to their layered structures.
Conclusion, These physical properties are critical tools in identifying minerals in the field and in laboratory settings. Although some properties like color may vary, a combination of these tests usually leads to accurate identification.
Chemical Properties of Minerals
Chemical properties of minerals are characteristics that describe how a mineral interacts with other substances, its composition, and its internal structure. These properties often involve changes to the mineral's chemical structure, such as during chemical reactions or decomposition. Here are some key chemical properties of minerals:
Chemical Composition
Each mineral has a specific chemical formula, such as SiO₂ for quartz (silicon dioxide) or NaCl for halite (sodium chloride). Variations in chemical composition can result in different minerals with similar properties.
Solubility
Solubility describes how readily a mineral dissolves in water or other solvents. It is a key property influencing how minerals break down and deposit in nature.
Types of Mineral Solubility
Highly Soluble Minerals: Halite (NaCl): Halite is highly soluble in water, forming sodium (Na⁺) and chloride (Cl⁻) ions in solution.
Sparingly Soluble Minerals: Gypsum (CaSO₄·2H₂O): Has limited solubility in water, releasing calcium and sulfate ions.
Insoluble Minerals: Quartz (SiO₂): Insoluble in water under normal conditions, it remains stable and resists dissolution even in harsh environments.
Fluorescence
Fluorescence is the ability of certain minerals to glow when exposed to ultraviolet (UV) light. The fluorescence is caused by impurities (activators) in the mineral that emit visible light when excited by UV radiation.
 |
Fluorescent fluorite mineral demonstrating its unique optical property under ultraviolet light. |
How to observe it: To test for fluorescence, expose the mineral to a UV light source in a dark environment and observe if the mineral emits a visible glow. UV lights used include both longwave (blacklight) and shortwave UV lamps.
Examples:
- Fluorite: Often glows in a variety of colors, most commonly blue or purple.
- Calcite: May glow in red, pink, or orange colors.
- Willemite: A zinc ore mineral that fluoresces bright green.
Reaction to Acid
The reaction of certain minerals to acid, typically dilute hydrochloric acid (HCl), can be used as a diagnostic property. When certain minerals react with acid, they effervesce (fizz), releasing carbon dioxide gas.
How to Test: A drop of dilute HCl is placed on the mineral surface, and the reaction is observed. Carbonate minerals, in particular, react by fizzing or bubbling.
Examples:
- Calcite: Fizzes vigorously when exposed to dilute HCl.
- Dolomite: Reacts weakly with cold HCl, but reacts more vigorously when the acid is warmed or the mineral is powdered.
- Malachite: A copper carbonate mineral that fizzes when exposed to acid.
Radioactivity
Radioactivity in minerals refers to the emission of particles from unstable atomic nuclei, typically from elements like uranium, thorium, or potassium. This radiation can be detected using instruments such as Geiger counters.
Some minerals contain radioactive elements like uranium, thorium, or potassium, which naturally undergo radioactive decay. This decay releases radiation (alpha, beta, or gamma rays) as the unstable isotopes break down into more stable forms. Radioactive minerals are used in dating geological formations, as the rate of decay (half-life) is consistent for specific isotopes.
Examples:
- Uraninite (UO₂): A highly radioactive mineral that contains uranium and is used in nuclear energy production.
- Thorite (ThSiO₄) and monazite are also radioactive minerals due to their thorium content.
- Potassium Feldspar contains trace amounts of the radioactive isotope potassium-40 (K-40).
Flammability
Flammability refers to a mineral’s ability to ignite and burn when exposed to heat or a flame.
Most minerals, especially those composed of inorganic elements, are non-flammable, meaning they do not easily ignite or sustain combustion. However, certain minerals containing organic components or elements that react with oxygen can burn under specific conditions. Flammable minerals often contain sulfur, carbon, or other reactive elements. Flammability is a key consideration in industries dealing with mineral processing, especially when burning materials like coal or sulfur minerals.
Example:
- Coal: A flammable mineral composed mainly of carbon and organic compounds, it combusts easily when exposed to heat, releasing energy as fuel.
- Sulfur (S): While it is a native element, sulfur is highly flammable and burns with a blue flame, releasing sulfur dioxide (SO₂) gas.
Decomposition/Thermal Stability
Decomposition or thermal stability describes a mineral's ability to remain intact or break down when exposed to heat.
Decomposition: Certain minerals break down or undergo chemical changes when heated to specific temperatures. This decomposition usually results in the loss of volatile components (like water, carbon dioxide, or sulfur gases) or a complete breakdown into simpler substances. For instance, carbonates like calcite (CaCO₃) will decompose at high temperatures, releasing carbon dioxide gas (CO₂) and leaving behind calcium oxide (CaO or quicklime).
Thermal Stability: Some minerals are more thermally stable and require very high temperatures to decompose. Quartz (SiO₂) is quite stable at high temperatures and does not decompose easily, which makes it valuable in high-temperature applications.
Hydration and Dehydration
Hydration is the process in which a mineral absorbs water molecules into its structure, while dehydration is the loss of water from a mineral's structure.
Some minerals contain water molecules within their crystal lattice, either as part of their composition or as loosely bound water. These minerals can gain or lose water depending on environmental conditions (e.g., humidity or temperature).
Hydration occurs when water is absorbed and incorporated into the mineral structure, often changing its form or composition. Dehydration happens when water is lost, typically under high temperatures, which can change the mineral’s chemical formula or lead to a new mineral form.
Example:
- Gypsum (CaSO₄·2H₂O): This mineral contains water in its structure. When heated, it loses water to become anhydrite (CaSO₄).
- Opal: Contains water in its structure, and the loss of water can cause cracking and changes in its appearance.
Oxidation
Oxidation refers to the chemical process where a mineral reacts with oxygen, leading to a change in its chemical composition, usually forming oxides or hydroxides.
Many minerals, especially those containing iron, can undergo oxidation when exposed to air or moisture. For instance, pyrite (FeS₂) often oxidizes to form iron oxides (rust), such as hematite (Fe₂O₃) or limonite (FeO(OH)).
 |
Cluster of pyrite crystals. The outside is rusty because the iron sulfide has oxidized to iron oxide. |
This chemical change can alter the appearance of a mineral, changing its color or texture. Oxidation often happens in the presence of water and oxygen, contributing to weathering and the breakdown of rocks over time.
Example:
- Pyrite (FeS₂) oxidizing into limonite (FeO(OH)).
- Copper oxidizing to form copper oxides, such as cuprite (Cu₂O), which gives a greenish patina.
Electrical Conductivity
Electrical conductivity refers to the ability of a mineral to conduct electric current. This property depends on the mineral’s atomic structure and the presence of free-moving electrons or ions. Minerals with metallic bonds, where electrons are free to move, are typically good conductors of electricity.
For example, metals like copper and gold exhibit high electrical conductivity because their atoms share a pool of free electrons, allowing the easy flow of electric current. In contrast, minerals with ionic or covalent bonds (e.g., quartz) are usually poor conductors or insulators because their electrons are tightly bound to the atoms and cannot move freely.
Thermal Conductivity
Thermal Conductivity is the ability to conduct heat. Minerals that can efficiently transfer heat are considered good thermal conductors. This property is related to the vibration of atoms and the transmission of these vibrations (heat) through the mineral's structure. Like electrical conductivity, metals tend to be good conductors of heat due to their free-moving electrons. Non-metallic minerals, such as diamond, can also have high thermal conductivity due to their strong and orderly atomic structures, even though they are electrical insulators.
Electrochemical Properties
Electrochemical properties of minerals refer to their ability to conduct or store electrical charges, or their behavior in electrochemical reactions (such as in corrosion or redox reactions).
Some minerals are conductive, allowing electrons to flow through them, which makes them important in industrial applications. For example, graphite and native metals like copper and gold are good conductors of electricity.
Additionally, minerals with electrochemical properties can be involved in redox (reduction-oxidation) reactions, where they either gain or lose electrons. These properties are essential in processes like corrosion or in batteries where minerals like galena (PbS) and sphalerite (ZnS) can participate in electrochemical reactions.
Example:
- Graphite is a good conductor of electricity.
- Galena (PbS) participates in electrochemical reactions in lead-acid batteries.
pH Sensitivity
Some minerals change their chemical structure or properties when exposed to environments with different pH levels (acidic or basic). Minerals may dissolve, react, or change form depending on the pH of their surroundings.
The chemical structure of some minerals is sensitive to changes in pH. In acidic environments (low pH), certain minerals may dissolve or react more readily, while in basic environments (high pH), different reactions may occur. These reactions can alter the mineral’s surface, color, or stability. pH sensitivity is particularly important in processes such as weathering and soil formation, as acidic rainwater can dissolve minerals, releasing ions into the environment.
Example:
- Calcite (CaCO₃) reacts with acids, such as hydrochloric acid, to produce carbon dioxide gas (CO₂) and dissolve. This is why calcite "fizzes" when in contact with acids.
- Feldspar can weather into clay minerals in slightly acidic conditions due to its sensitivity to pH.
Melting Point
The melting point is the temperature at which a mineral transitions from a solid to a liquid state.
Different minerals have distinct melting points depending on their chemical composition and atomic structure. Minerals with strong bonds (e.g., covalent bonds) generally have higher melting points, while those with weaker bonds (e.g., ionic bonds) melt at lower temperatures. Understanding the melting points of minerals is crucial in geological processes like magma formation and in industrial applications like metal smelting.
Example: Quartz (SiO₂) has a high melting point of about 1,710°C (3,110°F) due to its strong covalent bonds.
In summary, physical properties are observable characteristics that don't involve altering the mineral's structure, while chemical properties involve changes in composition or chemical reactions. Both sets of properties are crucial for identifying minerals and understanding their behavior in different environments.
Read also:
How to Identify Minerals in 10 Steps (Photos)
12 Most Common Minerals on Earth
The Complete Classification of Minerals
How to Identify Common Minerals








