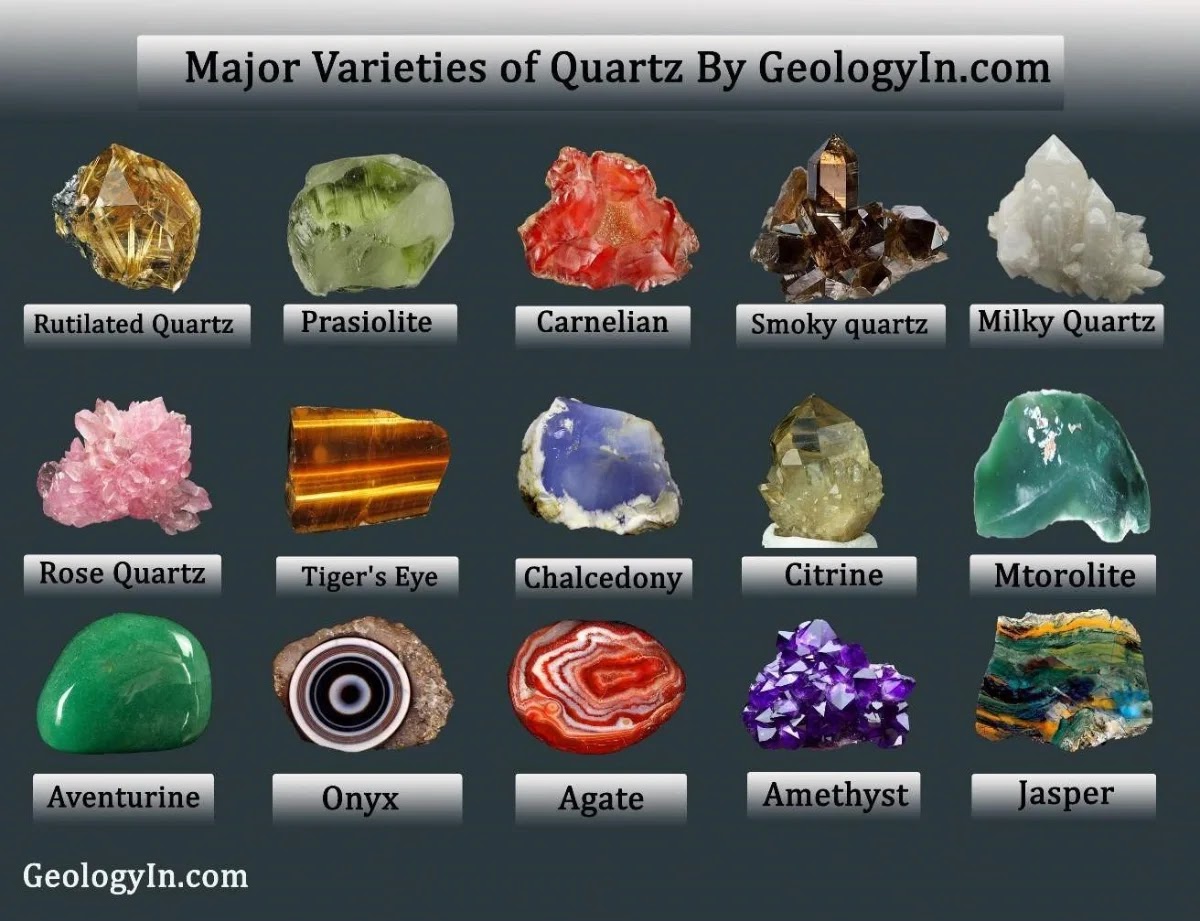
What Causes the Color of Amethyst
The purple color of amethyst is primarily caused by trace amounts of iron (Fe³⁺) incorporated into the quartz crystal lattice, combined with prolonged exposure to natural radiation. This process alters the electronic structure of the crystal, forming color centers—defects in the crystal lattice that absorb specific wavelengths of light and give amethyst its distinctive purple hue.
Initially, during crystal growth, some silicon atoms in the quartz (SiO₂) structure are replaced by iron atoms. Primarily, iron in the ferric state (Fe³⁺) substitutes for silicon atoms within the tetrahedral framework of the quartz structure. The mere presence of this substituted iron does not inherently produce the purple color.
 |
| A vibrant purple amethyst crystal cluster with natural facets. |
The crucial factor is prolonged exposure to natural gamma radiation. This radiation typically emanates from the decay of radioactive isotopes (like potassium-40 or elements in the uranium/thorium decay chains) present in the host rocks surrounding the quartz deposit.
The ionizing radiation provides sufficient energy to alter the electronic state of some of the iron ions within the lattice. Specifically, radiation can cause an electron to be removed from the electron cloud of a lattice Fe³⁺ ion, effectively oxidizing it to a higher valence state, often described as Fe⁴⁺. This specific ionic configuration (Fe⁴⁺ within the quartz lattice) acts as a "color center."
This newly formed Fe⁴⁺ color center has the property of strongly absorbing specific wavelengths of visible light. Its primary absorption bands are in the green and yellow regions of the spectrum (around 545 nanometers), with another significant absorption in the near-ultraviolet (around 357 nanometers).
When white light enters the amethyst crystal, the green and yellow wavelengths are preferentially absorbed by these color centers. The remaining wavelengths, predominantly in the blue and red parts of the spectrum (which combine to make violet/purple), are transmitted through the crystal or reflected off it. This transmitted/reflected light is what reaches the observer's eye, resulting in the perception of the amethyst's purple hue.
The depth and intensity of the color depend on several factors:
- Iron concentration: More iron can lead to deeper coloration, although too much may produce undesirable brownish tones.
- Radiation Dose: The amount and duration of radiation exposure determine how many potential iron sites are converted into active color centers. Insufficient radiation will result in pale coloration, even if iron is present.
- Presence of other trace elements: Ions of large ionic radius or other transition metals can influence the coloration process, even when iron content is low.
The color can fade with prolonged exposure to sunlight or heating, which disrupts these delicate color centers.
Amethyst occurs in hues ranging from light lilac to deep violet. Some specimens exhibit red or blue secondary hues, contributing to their visual appeal. The finest quality, known as "Deep Siberian," features a primary purple hue (75–80%) with subtle blue (15–20%) and red tones, depending on lighting.
Why and How Amethyst Loses Its Color
Amethyst can lose its characteristic purple color when exposed to certain environmental factors, primarily heat, light, or excessive radiation. These conditions disrupt the color centers within the quartz crystal lattice, which are responsible for the gemstone’s hue. The loss or alteration of color in amethyst is primarily caused by heat and exposure to intense radiation.
1. Heat-Induced Color Changes
When amethyst is heated to temperatures between 300 and 500 degrees Celsius, the iron-based color centers destabilize, causing the purple color to fade. Depending on the temperature and duration, the gemstone may turn yellow, orange, brown, or become colorless. This process is often irreversible in natural settings.The specific color outcome depends on both the temperature reached and the duration of heating. In some cases, heat-treated amethyst is deliberately converted into citrine or prasiolite (green quartz), which are also used as gemstones.
2. Radiation Exposure
Exposure to intense light, particularly ultraviolet (UV) light from sunlight or artificial sources, can also fade amethyst. UV radiation gradually breaks down the color centers, reducing the purple intensity over time. Similarly, high-energy radiation, such as X-rays, can alter or destroy these centers, leading to partial or complete color loss.
Conversely, controlled irradiation can artificially deepen amethyst’s purple color by enhancing the formation of color centers, though this is typically done in laboratory settings. To preserve amethyst’s natural color, it should be stored in a cool, dark environment, away from prolonged light or heat sources.
 |
| What Causes the Color of Amethyst? |
Tips for preventing your amethyst crystals from losing their color:
- Store your amethyst crystals in a cool, dark place.
- Avoid exposing your amethyst crystals to direct sunlight or other sources of ultraviolet light.
- Avoid heating your amethyst crystals.
- Be careful when cleaning your amethyst crystals, as some cleaning products can damage the color.


%20(1).webp)