Fluorite Colors: What Color is Fluorite
Fluorite is the world's most colorful mineral in the world, because of the enormous range of brilliant and even iridescent Colors it displays. Fluorite is the mineral form of calcium fluoride, CaF2. Fluorite belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
A crystal's color is dictated by the way light interacts with the chemicals in it, and by how these are bonded in an orderly structure, or lattice. Any impurities that work their way into fluorite's lattice can alter its apparent color. For example, manganese ions turn it orange.
Fluorite is Allochromatic, meaning that it can be tinted with elemental impurities. It is one of the most varied colored minerals in the mineral kingdom, and the colors may be very intense and almost electric.
 |
| Multicolored fluorite crystal with vibrant hues of purple, green, and blue, showcasing its natural geometric patterns and glossy surface. |
What Color is Fluorite
Fluorite is a mineral that comes in a wide range of colors. Its most common colors include:
- Purple (often light to dark shades)
- Green (from pale to deep green)
- Blue (light blue to deep blue)
- Yellow (bright yellow to golden)
- Colorless (clear or transparent)
- Pink
- Black
- Red
Some fluorite crystals can display multiple colors or even have a gradient (called a "Zoning" effect) where different colors appear in layers within the same crystal. This variability in color is due to the presence of trace elements or radiation exposure during the mineral's formation.
 |
| Why Fluorite Comes in Different Colors. Rainbow fluorite from Saxony, Germany. Photo: Wittig Minerals |
Why is Fluorite Colorful
Key factors responsible for the vibrant spectrum of fluorite
Impurities
While pure fluorite is colorless, tiny imperfections in the crystal's lattice structure play a significant role in its color. These imperfections can be:
Some of the most common impurities that cause fluorite to change color include:
- Manganese: This impurity causes fluorite to turn brown or black.
- Iron: This impurity causes fluorite to turn yellow, orange, or red.
- Copper: This impurity causes fluorite to turn blue or green.
- Yttrium: This impurity causes fluorite to turn purple.
- Zirconium: Zirconium impurities can cause fluorite to appear orange or red.
Radiation
Radiation: Exposure to natural radiation, like gamma rays or alpha particles, can also affect the color of fluorite. This radiation can create new color centers or modify existing ones, leading to a wider range of colors and even zoned patterns within the crystal.
Environmental Conditions
Environmental Conditions: The temperature, pressure, and chemical environment during the crystal's formation also play a role in its color. For example, fluorite formed in hydrothermal vents may have different colors than those formed in sedimentary environments.
Fluorite's ability to exhibit multiple colors within a single crystal, known as color zoning, is particularly fascinating. These zones can occur due to changes in the conditions of formation over time or variations in the concentration of coloring agents.
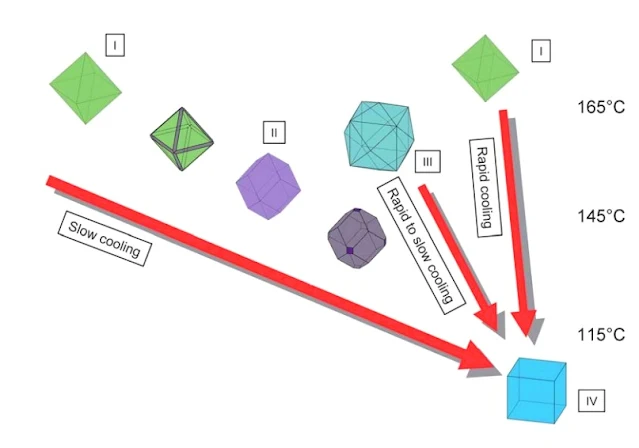 |
| Fluorite Different Colors and shapes ZIDAROVA et al. (1978) |
Diagram to show the variation of crystal morphology at
Nabburg-Wölsendorf as a function of temperature. Three lines of
evolution have been established based on the observation of the fluorite mineralization at Nabburg-Wölsendorf. They were interpreted in
terms of varying temperature gradients and correlated with temperatures for similar fluorite habits.
Rainbow Fluorite
A particularly striking variety of fluorite is "rainbow fluorite," which exhibits multiple colors in distinct bands or zones. This phenomenon occurs when different impurities or environmental conditions are present at different stages of crystal growth.
Fluorite forms in hydrothermal veins in the Earth's crust and in cavities in sedimentary rocks. Over the centuries, these fissures are constantly opening and closing, sometimes cutting off the fluids needed for fluorite to form. It's the subtle changes in the chemistry of these fluids that causes colour zoning in the crystals as they grow.
Some colors are deeply colored, and are especially pretty in large well-formed crystals, which Fluorite often forms. Sometimes coloring is caused by hydrocarbons, which can be removed from a specimen by heating. Some dealers may apply oil treatment upon amateur Fluorite specimens to enhance luster.
 |
| Rainbow Fluorite. Photo: Joe Kienle |
Fluorite Properties
Composition: Fluorite is the mineral form of calcium fluoride, with the chemical formula CaF₂.
Physical Properties
- Color: Fluorite comes in a stunning array of colors, including purple, green, blue, yellow, and colorless. The wide variety of colors is due to impurities in the crystal lattice.
- Transparency: Fluorite can be transparent to translucent.
- Luster: The luster of fluorite can be vitreous, meaning it has a glassy shine, or greasy.
- Crystal System: Fluorite crystals belong to the isometric crystal system, which means their crystals have three equal axes at right angles to each other.
- Streak: The streak of a mineral is the color of its powder. Fluorite's streak is white.
- Hardness: Fluorite has a hardness of 4 on the Mohs scale, which means it can be scratched by a knife but not by your fingernail.
- Cleavage: Fluorite has perfect cleavage in four directions, meaning it can be easily broken along these planes.
- Fracture: The fracture of fluorite is conchoidal, meaning it has smooth, curved breaks.
- Habit: Fluorite can be found in cubic, massive, or granular habits.
- Density: The density of fluorite is 3.18 g/cm³.
- Tenacity: Fluorite is brittle.
- Solubility: Fluorite is slightly soluble in hydrochloric acid.
- Magnetism: Fluorite is nonmagnetic.
Optical Properties
- Fluorescence: Fluorite may fluoresce blue, green, or white under shortwave ultraviolet light. This means that the mineral absorbs ultraviolet light and then emits visible light of a different color.
- Pleochroism: Pleochroism is the property of a mineral that exhibits different colors depending on the direction from which it is viewed. Fluorite can exhibit weak to distinct pleochroism.
- Refractive Index: The refractive index of a mineral is a measure of how much light is bent as it passes through the mineral. The refractive index of fluorite is 1.434 - 1.436.
Additional Properties
- Inclusions: Fluorite may contain inclusions of other minerals, such as calcite, quartz, or hydrocarbons.
- Field Indicators: Fluorite is often associated with hydrothermal deposits or limestone.
- Associated Minerals: Some of the minerals that are commonly associated with fluorite include galena, sphalerite, calcite, and quartz.
 |
| Fantastic fluorite on dolomite from Elmwood Mine, Tennessee. Credit: Anton Watzl |
 |
| Stunning Fluorite crystal is from a southern Illinois mine. Photo: Matthew Webb @MineralSpecimen |
 |
| Splendid fluorite crystals with a quartz on them. |
 |
| Fluorite from Shangbao Mine Hunan Province China. Credit: Exceptionalminerals..com |
 |
| Fluorite from Aouli, Morocco. Photo © Egil Hollund |
 |
| Perfect fluorite balls. The fluorite is not treated with oil or water. Collection, Photo & © Egil Hollund |
 |
| Amber coloured fluorite on quartz from Annaberg-Frohnau, Erzgebirge, Saxonia, Eastern Germany. Photo": © Jesse Fisher |
 |
| Rare fluorite-slice from Wölsendorf, Bavaria! Collection, photo & © Gerhard Brandstetter |
 |
| Fluorite and barite. from Guxian, Landkreis Tongbai, Provinz Henan Photo & © Joaquim Callen |
 |
| Fluorite type second generation, a combination of the crystal forms of cube and rhombic dodecahedron. Collection, photo & © Andreas Schmid |
 |
| Octahedron fluorite from Riemvasmaak fluorite occurrences, Kakamas District, Northern Cape Province, South Africa. Collection, photo & © Albert Russ |
 |
| A botryoidal Fluorite! from Guxian, Xinyang Präfektur, Henan, China. Photo: Roland Noack |
 |
| Polished Rainbow Fluorite from Hunan Province, Hunan Province, China |
 |
| banded Fluorite is just bands of different Colors. Photo by Joanne Dusatko. |
 |
| Rainbow Fluorite. Photo by The Crystal Spirit |

%20(1).webp)