Weathering and Soil
 |
| Weathering and Soil |
- Mechanical weathering is
the physical fragmentation of rock
- Chemical weathering is
the chemical alteration of mineral
- Mechanical weathering increases the exposed surface
area of rock on which chemical weathering can occur.
- Chemical weathering effects different minerals at
different rates. This weakens the fabric of the rock facilitating
mechanical weathering.
Mechanical
Weathering Processes
Frost wedging – in the daily freeze-thaw cycle at high altitudes, water seeps into cracks, freezes and expands extending the crack, then melts and seeps deep into the newly lengthened crack as the cycle repeats.
Thermal expansion – even in climates that do not experience daily freeze-thaw cycles, daily temperature cycles expand and contract the rocks causing fracturing.
Unloading – many rocks, for example plutonic igneous rocks, form deep in the crust. When they are exposed as the surface by the removal of overlying rocks by erosion, the pressure on the rock is reduced causing it to expand and crack. Rocks like granite often crack in concentric layers (like an onion) resulting in a process known as exfoliation. This causes granite domes such as Half Dome in Yosemite, Enchanted Rock in Texas, and Stone Mountain in Georgia.
Organic activity – plant roots, burrowing animals, etc.
Abrasion – corners are fragile things on rocks like on furniture. As rocks are transported, they abrade against one another, removing corners first, then edges. Thus, as transport time and distance increases, rock fragments become rounded (not necessarily spherical) as corners and edges are removed.
Chemical
Weathering Processes
Solution (or dissolution) – This is the dissolving of soluble minerals in water or weak acids. Soluble minerals are generally ionically bonded minerals such as calcite and halite. Silicate minerals are not subject to solutioning. Solutioning requires considerable amounts of water to remove much material. It is thus most effective in wet climates. Solutioning is very effective on carbonate rocks.
Oxidation - This reaction effects iron bearing minerals, forming iron oxides. Iron oxides are strong coloring agents and give many rocks their reddish or tan coloration.
Hydrolysis - this reaction is the most important weathering reaction because it effects the silicate minerals. Silicates with ionically bonded metal ions (everything except quartz) are weathered by this reaction. In hydrolysis, the H+ ion from water or weak acid works its way into the mineral structure due to its very small size (it is a nucleus with no electrons). Because the H+ ion is so reactive, it dislodges other metal ions and causes the chemical breakdown of the crystal structure. The most important byproduct of hydrolysis are clay minerals. Because silicates are the most abundant minerals in the crust, clay minerals are the most abundant byproduct of weathering.
Rates of Weathering
In general, the wetter the climate,
the faster weathering occurs
In wet climates, carbonates(solutioning) usually weather faster than silicates (hydrolysis) since solutioning requires more water than hydrolysis. The opposite is true in arid and semi-arid climates where carbonates like limestones usually weather more slowly than carbonates. In central Texas, limestones weather slowly, thus the Alamo has not yet dissolved!
Mafic silicates weather more rapidly than felsic silicates. Thus the ferromagnesian silicates weather rapidly into clays and iron oxides. Feldspars weather more slowly. Quartz does not chemically weather.
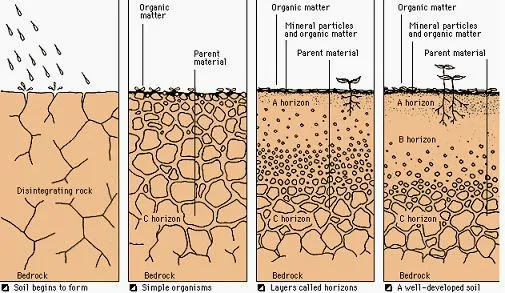
Soils
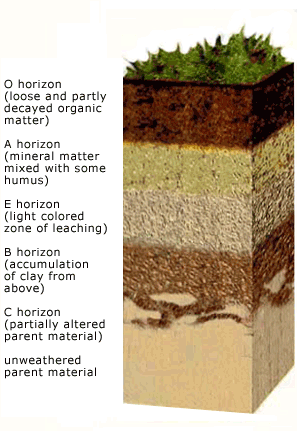
Soil Horizons
Soil-Forming Factors
- Climate-
rainfall is most important, temperature range is also important
- Parent rock -
controls the minerals available for soil
- Slope angle and aspect - steeper slopes generally mean thinner soils,
aspect controls plant growth and soil moisture levels.
- Time-
if an area is stipped by periodic windstorms or flooding, thick soil will
not endure
- Organic growth -
type and density of plant growth
Major Soil Types
Pedalfers
Pedalfers are soils which form in regions of moderately high rainfall and cool to moderate temperatures. These soils are rich in Al and Fe, hence the name PedAlFer. These soils are generally rich brown soils and underlie much of the eastern and midwestern US. The main process in forming this soil is moderate leaching. Most soluble ions (Ca, Na) are removed. Aluminum is present in clays that predominate in the B layer (subsoil) along with iron oxides.
Pedocals
Laterites
Laterites are thick red soils that develop in areas of high rainfall and temperature. Extensive leaching has removed all of the soluble ions as well as some of the clays, leaving mostly aluminum oxides and iron oxides. Laterites are nutrient poor soils. The fact that they underly many tropical rainforests attests to the fact that the nutrient cycles in tropical rainforests largely bypass the soil. Nutrients are recycled rapidly from decaying organic material back into living plants.
Symbiotic plants often grow directly on other plants rather than on the soil. Cutting, or worse burning, rainforests not only removes an important source of oxygen, and sink for carbon dioxide, but leaves behind land that is unsuitable for most forms of agriculture. Laterites dry to brick hard consistency and are used as building material. Aluminum rich laterites are known as bauxite, and are the primary ore for aluminum. Since extracting aluminum from bauxite is relatively expensive, recycling of aluminum has proven cost effective and is therefore one of the most successful examples of recycling.








%20(1).webp)