Colors of Minerals and Gemstones
One of the most important physical properties of minerals, reflecting the nature of the interaction of the electromagnetic radiation of the visible region with the electrons of the atoms, molecules, and ions of the crystals and with the electron system of the crystal as a whole. In mineralogy, color is one of the primary diagnostic properties of natural compounds, of great importance in geological prospecting for the identification of minerals.
The color of gems and semiprecious stones is one of their main qualitative (gem) characteristics. A distinction is made between the color of minerals in individual crystals and lumps of ore, the color of minerals in transparent thin sections (under the microscope), the color of minerals in polished sections (in reflected light), and the color of a mineral’s streak (the color of the fine powder of the mineral).
Causes of Gemstones Colors
Absorption of Light: When white light strikes a mineral or gemstone, certain wavelengths are absorbed while others are reflected. The reflected colors are what we perceive.
Impurities and Defects: The presence of trace elements or imperfections in the crystal structure can affect how light interacts with the material, causing specific colors to be absorbed or enhanced. For example, chromium impurities in ruby contribute to its red color.
Crystal Structure: The arrangement of atoms in the crystal lattice can influence how light is transmitted and reflected, leading to specific colors. For instance, the hexagonal structure of corundum (including ruby and sapphire) contributes to their vibrant hues.
Structural Defects: Certain defects like vacancies or substitutions within the crystal lattice can create color centers, trapping and releasing light energy in specific ways, resulting in distinct colors.
 |
| Color of Minerals. Colorful Garnet Family: Almandite, Blue Garnet, Demantoid, Hessonite, Malaia Garnet, Mali Garnet, Pyrope, Rhodolite, Spessartite, Tsavorite.: Garnet Gemstone,© Wowoon Company |
Types of Color in Minerals and Gemstones
Three main groups of minerals are identified on the basis of the property of color: idiochromatic, allochromatic, and pseudochro-matic.
Idiochromatic
Idiochromatic minerals are minerals that possess inherent color due to their chemical composition. In simpler terms, the elements that make up the mineral structure directly contribute to its specific color.
The color in idiochromatic minerals arises from the absorption or emission of specific wavelengths of light by certain transition metals within the mineral's crystal structure. These transition metals possess electrons that can readily jump between energy levels when exposed to light, resulting in the absorption or emission of specific colors.
In idiochromatic mineral, the color is due to electron transfers between different ions, namely, charge transfers. This includes the transfer between a metal ion and ligands and the transfer between differently charged metal ions. Examples are minerals of trivalent iron (the charge transfer O2 → Fe3+); the chromates, vanadates, and molybdates, such as crocoite, vanadinite, and wulfenite (the transfer O2 → Cr6+, V5+, Mo6+); and minerals that at the same time contain the differently charged ions Fe2+ and Fe3+, such as cordierite, vivianite, and aquamarine.
- Beryl and Fe++ = Aquamarine (blue)
- Beryl and Fe+++ = Heliodor (yellow)
- Beryl and Mn++ = Morganite (pink)
- Beryl and Cr+++ = Emerald (green)
Color associated with ions of the transition metals—Ti, V, Cr, Mn, Fe, Co, Ni, and Cu—is typical of emerald, ruby, rubellite, rhodonite, chrysolite, and malachite. The lanthanides and actinides are chromophores of minerals of the rare-earth elements and uranyl. Their color is due to the transfers of electrons between the d- and f-levels of the chromophore ions.
Color caused by radioactivity is related to the formation of electron-hole color centers by the action of natural ionizing radiation, for example, the dark blue and purple color of halite and fluorite and the yellow and smoky color of quartz and calcite.
Examples: Some well-known examples of idiochromatic minerals include:
- Malachite (Green) - Copper (Cu) is the chromophore, giving malachite its characteristic green color.
- Azurite (Blue) - Copper (Cu) is again the chromophore, but in a different chemical environment within the azurite structure, leading to a blue color.
- Cinnabar (Red) - Mercury (Hg) is the chromophore, imparting a vivid red color to cinnabar.
Allochromatic
Allochromatic minerals are minerals that display a variety of colors due to the presence of impurities or imperfections within their crystal structure, rather than their inherent chemical composition. These "coloring agents" can significantly alter the mineral's appearance compared to its pure form, which would often be colorless.
A key characteristic of allochromatic minerals is the wide range of colors they can exhibit. The same mineral type can appear in various hues depending on the type and concentration of the coloring agents or structural defects present. For instance, quartz, an allochromatic mineral, can be colorless, pink (rose quartz due to manganese), yellow (citrine due to iron), or smoky gray (due to aluminum vacancies).
Examples: Many popular gemstones fall under the category of allochromatic minerals, including:
- Quartz (variety of colors based on impurities)
- Beryl (emerald - chromium; aquamarine - iron)
- Corundum (ruby - chromium; sapphire - iron, titanium)
- Topaz (various colors based on impurities)
- Spinel (variety of colors based on impurities)
Pseudochromatic
Pseudochromatic minerals are a category distinct from idiochromatic and allochromatic minerals. Unlike the other two, their color doesn't arise from the inherent chemical composition or impurities within the crystal structure. Instead, the color in pseudochromatic minerals is a result of light interacting with the mineral's physical structure in specific ways.
The captivating colors displayed by pseudochromatic minerals are due to phenomena like diffraction, scattering, interference, and refraction of light as it travels through the mineral's structure. These minerals often possess microscopic features that influence how light interacts with them, creating a play of colors or a specific color effect.
In pseudochromatic minerals the color is due to the diffraction and interference of light, as well as to the dispersion, refraction, and total internal reflection of incident white light. These phenomena are related to the structural features of mineral formations (regular alternation of phases of different composition in iridescent labradorites and peristerites and in sunstone [aventurine feldspar] and moonstone; the globular structure of opals) or to the structure of the surface layer of crystals (various types of tarnish, such as the iridiscent film on bornite, chalcopyrite, pyrite, and covellite).
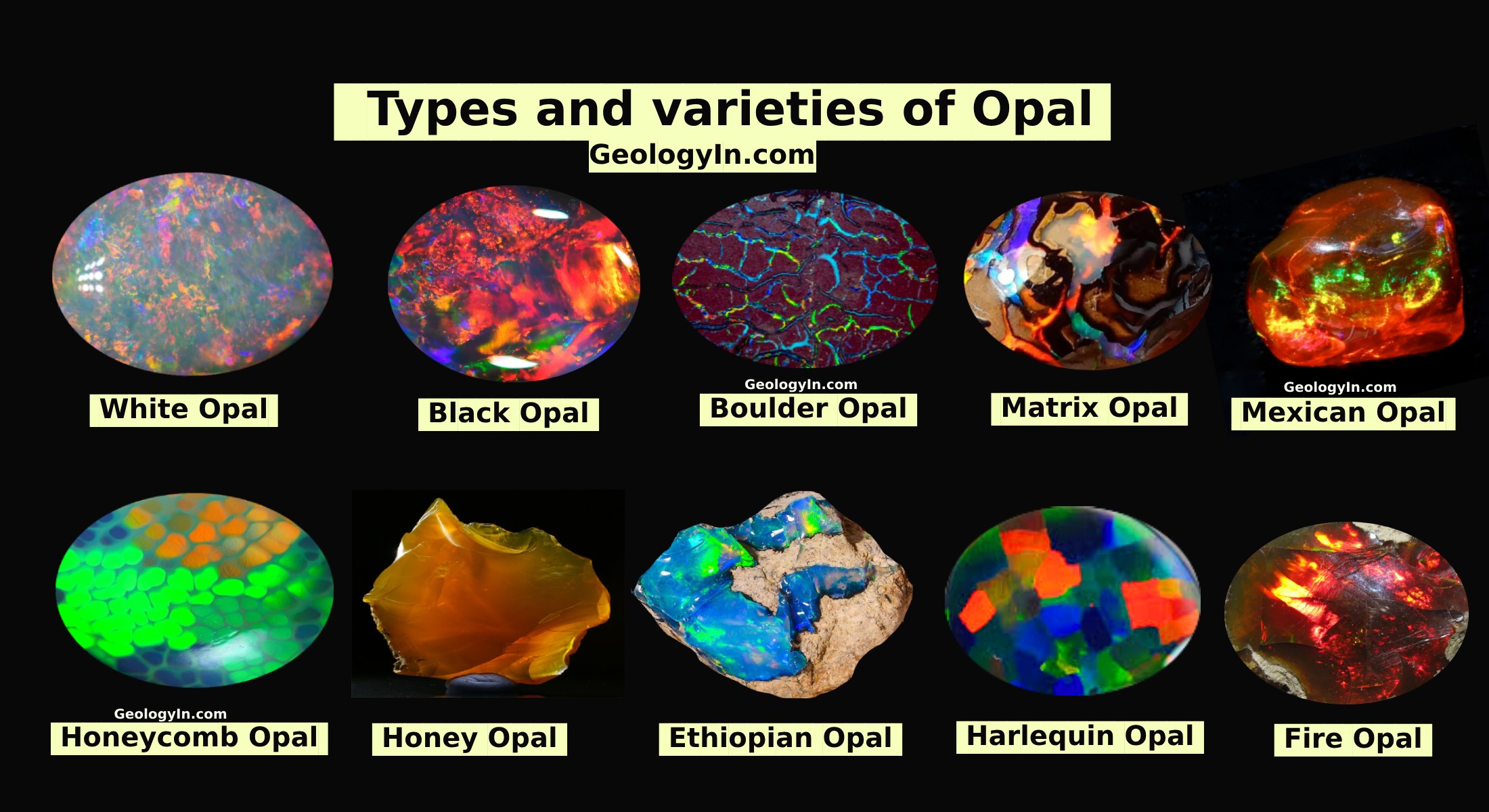
Examples: Some well-known examples of pseudochromatic minerals include:
- Opal (play of color due to diffraction)
- Labradorite (labradorescence due to light scattering)
- Moonstone (adularescence due to light scattering)
- Fire opal (play of color due to diffraction and interference)
The color of metallic and covalent compounds, such as native metals and sulfides and their analogs, is due to interzonal optical transfers of electrons and the related maximums of reflection (for example, the metallic colors of pyrite and gold) or is due to the fundamental absorption band (cinnabar, orpiment, cuprite).
 |
| Color of Minerals. Credit: Carina Rossner |
Minerals and Gemstones Color Description
A comparative evaluation is usually used in describing the color of minerals; the mineral’s color is compared to the color of some commonly known object or substance (indigo blue, apple green, lemon yellow, and blood red) or to mineral “color standards,” such as vermilion red and emerald green.
The colors of metals or alloys are used as standards for describing the color of ore minerals: tin white (arsenopyrite), steel gray (molybdenite), brass yellow (chalcopyrite), and copper red (native copper). Methods are being developed for an objective evaluation of the color of minerals, especially of gems, using standard colorimetric characteristics. Many minerals have the property of exhibiting different colors in different crystallographic directions, especially in polarized light, or changing their color with the color temperature of the radiative source illuminating them.
The study of the color of minerals provides information about the crystallochemical and genetic characteristics of minerals and is useful in the synthesis of high-quality analogs of natural gems.

%20(1).webp)